Recommendation for individuals using a screenreader: please set your punctuation settings to "most."
Descriptive Statements:
- Analyze different types of chemical reactions.
- Predict the outcomes of chemical reactions.
- Demonstrate knowledge of collision theory and factors that influence reaction rates.
- Analyze rate problems and experimental rate data.
Sample Item:
Which of the following products is formed by an esterification reaction between acetic
acid (CH3CO2H) and ethanol (CH3CH2OH)?
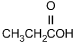
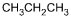
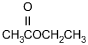
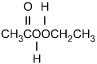
Correct Response and Explanation (Show Correct ResponseHide Correct Response)
C. This question requires the examinee to analyze different types of
chemical reactions. In the esterification reaction between acetic acid and ethanol, the
OH group from acetic acid and the H attached to the O in ethanol combine to form water.
The remaining portions of the acetic acid and ethanol molecules combine to form the ester,
ethyl acetate.
Descriptive Statements:
- Demonstrate knowledge of the concept of chemical equilibrium and the factors that influence chemical equilibrium.
- Apply Le Châtelier's principle to chemical systems.
- Solve problems involving equilibrium constants.
Sample Item:
Zn(s) + 2H+(aq) 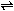 Zn2+(aq) + H2(g)Zn left parenthesis s right parenthesis plus 2H positive left parenthesis aq right parenthesis equilibrium arrow Zn superscript 2 positive left parenthesis aq right parenthesis plus H2 left parenthesis g right parenthesis
Zn2+(aq) + H2(g)Zn left parenthesis s right parenthesis plus 2H positive left parenthesis aq right parenthesis equilibrium arrow Zn superscript 2 positive left parenthesis aq right parenthesis plus H2 left parenthesis g right parenthesis
Which of the following is the equilibrium constant expression for the equation shown above?
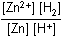
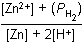
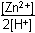
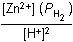
Correct Response and Explanation (Show Correct ResponseHide Correct Response)
D. This question requires the examinee to solve problems involving
equilibrium constants. When writing equilibrium constant expressions, pure solid and
pure liquid compounds are omitted and the pressure of gaseous compounds can be used in
place of concentration. The equilibrium constant expression for this reaction is equal
to the concentration of Zn2+(aq) × the pressure of H2(g)Zn superscript 2 positive left parenthesis aq right parenthesis times the pressure of H2 left parenthesis g right parenthesis each
raised to a power equal to its stoichiometric coefficient, divided by the concentration
of H+(aq)H superscript positive left parenthesis aq right parenthesis raised to a power equal to its stoichiometric coefficient.
Descriptive Statements:
- Analyze acids and bases according to how they behave and how they are defined.
- Determine the hydronium ion concentration, hydroxide ion concentration, pH, and pOH for acid, base, and salt solutions.
- Demonstrate knowledge of the relationship between molecular structure and acid strength and the relative strengths of acids and bases.
- Analyze buffer solutions qualitatively and quantitatively.
- Demonstrate knowledge of the principles and applications of acid-base titrations.
Sample Item:
Which of the following explains why nitric acid (HNO3) is a stronger acid than nitrous
acid (HNO2)?
- The additional oxygen present in nitric acid increases the polarity of the
O—H bond.
- The extent of ionization is directly related to molecular weight when comparing
related compounds.
- The anion formed by removing H+H positive from nitrous acid is more stable than the anion
formed by removing H+H positive from nitric acid.
- The O—H bond in nitrous acid is weaker than the O—H bond in nitric acid.
Correct Response and Explanation (Show Correct ResponseHide Correct Response)
A. This question requires the examinee to demonstrate knowledge of the
relationship between molecular structure and acid strength. The strength of an acid is
a function of its tendency to ionize. For oxoacids with the same central atom, acid
strength increases as the oxidation number of the central atom increases because of the
resulting increase in polarity of the O—H bond. The oxidation number of nitrogen in
HNO3 is +5positive 5 and in HNO2 it is +3positive 3, thus the O—H bond in HNO3 is more polar and ionizes more readily.
Descriptive Statements:
- Demonstrate knowledge of oxidation, reduction, oxidation numbers, and the balancing of oxidation-reduction equations.
- Analyze the components and operating principles of electrochemical cells and electrolytic cells.
- Solve problems involving electrochemical cells.
- Demonstrate knowledge of the applications of electrochemistry.
Sample Item:
Automobile mechanics measure the density of the electrolyte solution of lead storage
batteries to determine the amount of charge remaining. Which of the following statements
describes the cause of the change in electrolyte density as the battery's charge decreases?
- The sulfuric acid electrolyte is consumed and water is formed.
- Water evaporates and the electrolyte concentration increases.
- The lead and lead(IV) left parenthesis IV right parenthesis oxide migrate from the solution to the electrodes.
- Lead in the electrolyte solution precipitates out of the solution.
Correct Response and Explanation (Show Correct ResponseHide Correct Response)
A. This question requires the examinee to demonstrate knowledge of the
applications of electrochemistry. During the normal operation of a lead storage battery,
sulfuric acid is consumed and water is produced. The density of the electrolyte solution
is related to how much of each of these substances is present in the solution.

